President of India Droupadi Murmu launched first indigenously made-in-India anti-Cancer CAR-T cell therapy ‘NexCAR19’. With this, India became first developing country to have its indigenous CAR-T gene therapy.
About NexCAR19
- It is outcome of academia-industry partnership and has been developed thorough collaboration between IIT Bombay, Tata Memorial Centre and ImmunoACT.
- ImmunoAct is IIT-Bombay incubated company launched in 2018 to provide affordable access to novel autologous CAR-T cell therapies.
- It is world’s most affordable CAR-T therapy and puts India firmly on the Global Map of Advanced Cell and Gene Therapy.
- It has received approval from Central Drugs Standard Control (CDSCO) for the treatment of Relapsed-refractory (R/R) B- cell lymphomas and leukemia.
What is CAR-T cell therapy?
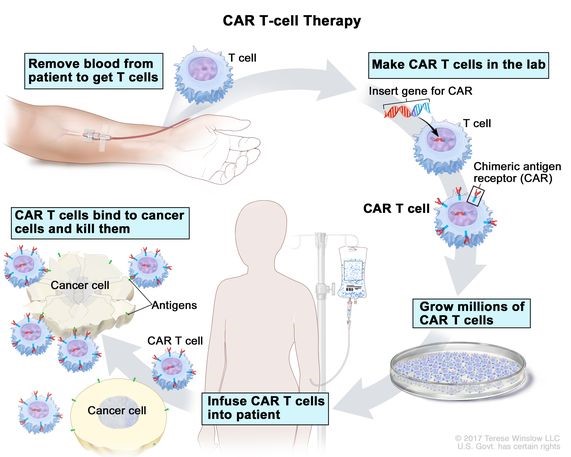
- CAR-T (Chimeric antigen receptor) therapy modifies immune cells, specifically T-cells, by turning them into potent cancer fighters known as CAR-T cells.
- T-cells are special cells (types of white blood cells) whose primary function is cytotoxic (i.e killing other cells).
- T cells are taken from patient blood and are changed in lab by adding a gene for a man-made receptor (called CAR).
- CARs are proteins that assist the T-cells to recognise and attach to a specific protein present on cancer cells. CAR-T cells are then given back to the patient.
- Benefits of the CAR-T Cell therapy: It can treat cancer for an extended period and has the potential to cure specific cancers completely.